FUGELS stands for Functionally Graded Electrodes for Long-Life Lithium-Sulfur Batteries
The FUGELS project aims to accelerate the maturation of lithium-sulfur batteries (LSBs) for future wide deployment in electrochemical-energy-storage (EES) applications. Unlike the Li-ion batteries which use transition metal oxides (like nickel manganese cobalt oxide) or iron phosphate (LFP) as the cathode, and graphite as the anode, the Li-S battery employs sulfur on the cathode, and lithium metal as the anode. Therefore, Li-S batteries are cheaper, and lighter compared to Li-ion batteries and do not observe the raw material supply risks (like Co which can only be found in certain location on earth). There are many potential benefits from this emerging battery technology that outweigh the scientific investments essential for clearing the hurdles on its way to EES market. In short, the LSBs have a short life span and suffer from a significant rate of aging. Here, the aging phenomena are multifaceted and different from those in state-of-the-art commercial lithium-ion batteries (LIBs).
Goals
In-depth quantitative understanding of aging dynamics at particle, electrode, and cell level
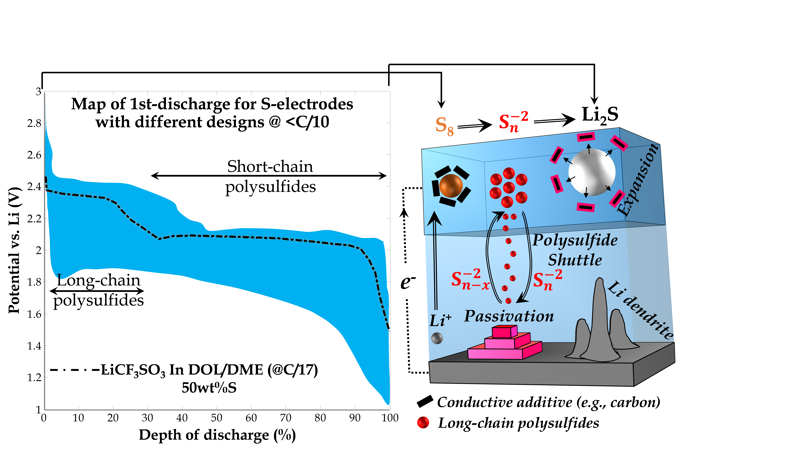
Premature end-of-life is the biggest obstacle to the maturation of lithium-sulfur battery technology. LSBs suffer from a significant rate of capacity loss and impedance increase compared to LIBs. The primary reasons for this rather fast aging rate are: insulating nature of the sulfur and discharge products (Li2S), expansion of the cathode volume upon discharge (~70%), lithium dendrite formation, and most importantly dissolution of the intermediate lithium polysulfide redox species into the electrolyte. These polysulfide species are mobile in the electrolyte and able to leave their local reaction zone in the cathode before complete conversion to the end-discharge product, i.e., Li2S.
Development of functionally graded particles and electrodes to mitigate aging.
The mobility of polysulfide species in the cell is recognized as one of the most important origins of aging. The physical/chemical confinement of polysulfide species in the cathode region with polysulfide trap centers (PS-trap) is one the most tied approaches to limit the mobility of polysulfide. With careful design considerations we aim to keep the polysulfides’ redistribution under control once they are inside the electrolyte. Such designs for LSBs are achievable by considering the complex electrochemistry and transport properties of the polysulfide species.
Project Partners

UHasselt-EE Electrochemical Engineering (EE) group at Hasselt University co-rdinates the FUGLES project. The group focusses on the fundamental engineering aspects of electrochemical systems such as advanced batteries, electrolyzers, and fuel cells. EE is is part of the Energy Storage research cluster at the Institute for Materials Research (imo-imomec) and is a partner at Energyville.

UHasselt- DESINe The main activity of the group of “Design and synthesis of inorganic materials” is the study of environmentally friendly, chemical methods for the preparation of high-tech, nanostructured inorganic materials such as solution-based synthesis routes, including water-based and non-water-based sol-gel methods, hydro-/solvothermal synthesis, combustion synthesis, co-precipitation, thermal decomposition synthesis, etc. DESINe is a partner in FUGLES on development of advanced active materials for the Li-S batteries. The group is also affiliated with Energyville.

imec-Estore Interuniversity Microelectronics Centre is an international research & development organization, active in the fields of nanoelectronics and digital technologies. The Energy Storage group at imec has extensive experience on thin film deposition techniques and advanced characterization methods for application in electrochemical energy storage devices and play a critical role in the FUGLES project. Imec-Estore is also a partner in Energyville.

Ghent University- CoCooN Group CoCooN or the group of “Conformal Coating of Nanomaterials” is a research group at the science department of Ghent University. Their research can be grouped in 3 main topics: • Research methods for thin film deposition: atomic layer deposition, combinatorial materials screening, coating of particles • Research methods and facilities for thin film characterization: in situ characterization, integrated glovebox and UHV line, synchrotron experiments • Applications based on thin films: nano electronics, batteries, electrocatalysis, etc. The invaluable experience of the CoCoon group makes them an indispensable part of the FUGELS project.

EMAT- University of Antwerp Electron Microscopy for Materials Science (EMAT) group is one of the leading electron microscopy centers in the world and has a vast expertise in both fundamental and applied electron microscopy. EMAT has several state of the art electron microscopes including two aberration corrected, high end FEI-Titan instruments, a dual beam FIB, an environmental SEM, and etc. EMAT helps in the FUGELS project with their expertise in materials characterization.

VITO Vlaamse Instelling voor Technologisch Onderzoek or Flemish Institute for Technological Research is an independent research organization in the field of cleantech and sustainable development. The group of Sustainable Materials from VITO is partner in FUGELS and are sharing their knowledge on advanced coating methods like atmospheric plasma technology to help overcome the challenges in Li-S batteries.
Industry advisory board











Supported by


