











The research activities of the DSOS group are oriented on the development of synthetic routes for novel organic semiconductors - conjugated polymers as well as small molecules - and their implementation in organic electronics and advanced healthcare.


Our group is mainly focusing on material synthesis, with a particular emphasis on structural purity and profound material characterization, and possesses all synthesis and characterization facilities to be competitive on an international level. Moreover, the integration in the Institute for Materials Research (imo-imomec) provides access to state-of-the-art device fabrication and (optoelectronic) characterization tools. All projects are conducted in close collaboration with Materials Physics colleagues to unravel the processes underlying device performance.

Since 2021, the DSOS group has been working on materials for organic electrochemical transistors. These devices consist of a semiconducting polymer channel, capable of conducting ions as well as electronic charges. This interesting characteristic gives OECTs the ability to connect the soft and ionic world of biology with the hard and electronic world of solid-state semiconductor physics. Even though the field is still young, it is believed that OECTs will revolutionize electronic healthcare through applications in biosensors, electrophysiology, brain-computer interfaces (BCI’s), etc. To achieve mixed conduction, hydrophilic analogs of established semiconducting polymers are synthesized by incorporating various hydrophilic side chains on (p- or n-type) polymer backbones.
In the EU-funded MITICS project, we focused on the bioelectronic applications of OECTs (notably brain-computer interfaces), while the FWO WEAVE project (in collaboration with the Vandewal and Banerji groups) deals with the more fundamental understanding of electronic-ionic interactions for high-performance and stable OECTs.

Over the past decade, a vast number of organic semiconducting materials have been developed to exploit the light emission principle of thermally activated delayed fluorescence (TADF). This concept enables the realization of unique optical and electronic properties arising from the efficient thermal equilibration of the lowest singlet and triplet excited states of organic fluorophores. As a result, TADF-based organic light-emitting diodes (OLEDs) show significantly upgraded device performances, comparable to those provided by traditional rare metal complexes.
In the DSOS group, advanced TADF materials are designed, synthesized, and characterized by a rational approach combining complementary computational and synthetic materials expertise. We are particularly interested in extending the TADF emission range to the near-infrared (NIR). In parallel, we also explore similar materials for triplet-triplet annihilation (TTA). On top, we have started the development of chiral small-molecule and polymer emitters showing circularly-polarized luminescence, aiming at combined high dissymmetry factors and external quantum efficiencies in the resulting OLED devices.

Over the past 15 years, the DSOS group has been very active in the field of organic/polymer solar cells. These organic thin-film photovoltaics have demonstrated strong potential as an innovative source of renewable energy, adding appealing features to classical solar cell technology, in particular in terms of architectural freedom (flexibility, reduced weight, color, semitransparency), low-light performance, and low-cost (high-throughput) large-area production.
Despite the impressive progress in the field over the last years, especially in terms of device efficiency (nowadays exceeding 20%), further dedicated research efforts are still required to enable successful market entrance. In this respect, the DSOS group addresses all three parts of the 'photovoltaic triangle', i.e., efficiency, stability, and cost, with dedicated (Ph.D.) research projects, pursuing the rationalization of structure-property relations. Nowadays, we mainly focus on materials for the blue and red subcells of (RAINBOW) tandem solar cells, novel conjugated block copolymers for single-component OPVs, and the implementation of continuous flow chemistry to improve material and device reproducibility, in strong collaboration with the OOE group (Prof. K. Vandewal) at imo-imomec.

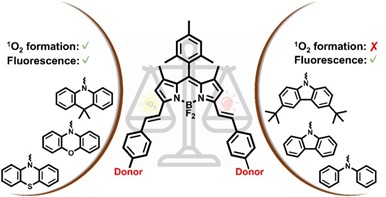
Over the years, the DSOS group has broadened its scope of activities to organic semiconducting materials for advanced healthcare, for which we strongly collaborate with the NSI (Nanobiophysics and Soft matter Interfaces) group at imo-imomec. From an initial focus on (luminescent) conjugated polymer nanoparticles for bioimaging, we moved on to push-pull (BODIPY) dyes for image-guided photodynamic therapy. The aim is to cover the whole research chain from computational materials design to (NIR-photoactive) photosensitizer synthesis and characterization, incorporation of the advanced photosensitizer in specifically designed nanoparticles, to studies of the cytotoxicity and intracellular dynamics of the nanoparticles in cells and biological tissues using advanced optical imaging techniques.

Hydrogen will likely play a key role in the future renewable energy mix, allowing transport and storage for an on-demand energy supply. Sustainable hydrogen generation can be achieved by photocatalytic water-splitting. Organic/polymer semiconductors are very attractive photocatalysts for this purpose, as they can be synthetically tuned to absorb a maximum amount of solar light while simultaneously retaining suitable energy levels to drive hydrogen evolution. However, they only recently gained notable attention, and a better understanding of the structural parameters and photophysics that determine the function of organic semiconductor heterojunction nanoparticles is required to optimize their performance.
In the DSOS group, we address this challenge by an integrated approach, combining tailored organic/polymer semiconductor synthesis, preparation and characterization of optimal nanoparticle formulations, detailed photophysical evaluation of the charge generation and separation processes, and standard hydrogen evolution measurements. The overall objective is to establish robust structure-property relationships – using different feedback loops – to maximize the solar-to-hydrogen conversion efficiency.

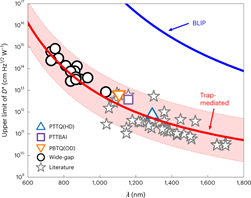
For UV-visible light detection, organic photodetectors already match and even surpass the performance of state-of-the-art inorganic photodetectors. Unfortunately, organic materials generally show limited absorption in the near-infrared (NIR) part of the spectrum. For this reason, we target novel NIR-absorbing materials for regular bulk heterojunction NIR-OPDs as well as organic cavity enhanced photodetectors, with the aim to elucidate the intrinsic limitations of NIR photodetection based on organic semiconductors (in the pursuit of a marketable technology).
We have recently shown that the main factor limiting the specific detectivity is non-radiative recombination, which is also known to be the main contributor to open-circuit voltage losses in OPVs. Based on this finding we concluded that OPDs have the potential to be a useful technology up to 2 μm, given that high external quantum efficiencies can be maintained at these low photon energies. To further elaborate on the fundamentals defining these limitations, we now focus on the influence of specific material imperfections on the material properties and device performance, in close collaboration with the OOE group (Prof. K. Vandewal) at imo-imomec.

The DSOS group has a long-standing interest in the synthesis and applications of porphyrinoid materials (push-pull porphyrins, corroles, …) and related BODIPY (boron dipyrromethene) dyes, specifically in relation to organic electronics and advanced healthcare applications. Detailed photophysical characterization of these oligopyrrolic chromophores is conducted in close collaboration with spectroscopists and theoretical chemists.














Nayak, S.; Caz, N.; Derveaux, E.; Smeets, S.; Cardeynaels, T.; Wolfs, E.; Adriaensens, P.; Maes, W.; Ethirajan, A., Reactive oxygen species responsive dextran-thioketal conjugate nanocarriers for the delivery of hydrophilic payloads Carbohydrate Polymers 2025, 356, 123375 – DOI: 10.1016/j.carbpol.2025.123375
















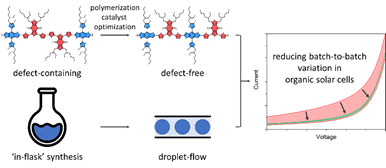


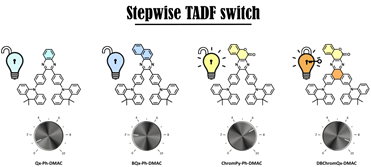

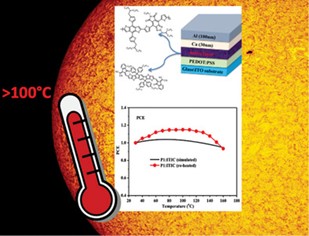


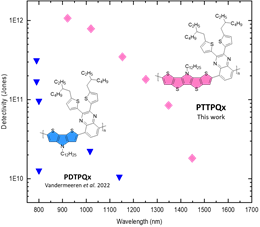







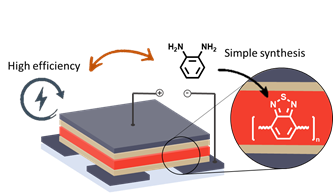
Valkeneers, K.; Vandewal, K.; Maes, W., “Benzothiadiazole-based push-pull copolymers – balancing synthetic complexity against organic solar cell efficiency” Org. Electron. 2022, 111, 106667 – DOI: 10.1016/j.orgel.2022.106667
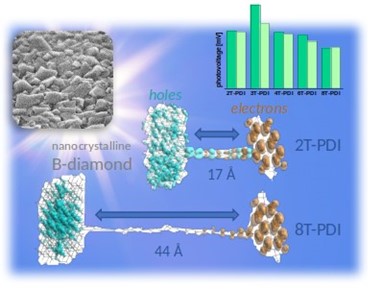
López-Carballeira, D.; Raymakers, J.; Artemenko, A.; Lenaerts, R.; Čermák, J.; Kuliček, J.; Nicley, S. S.; Kromka, A.; Haenen, K.; Maes, W.; Rezek, B., “Effect of oligothiophene spacer length on photogenerated charge transfer from perylene diimide to boron-doped diamond electrodes” Sol. Energy Mater. Sol. Cells 2022, 248, 111984 – DOI: 10.1016/j.solmat.2022.111984

Cardeynaels, T.; Etherington, M. K.; Paredis, S.; Batsanov, A. S.; Deckers, J.; Stavrou, K.; Vanderzande, D.; Monkman, A. P.; Champagne, B.; Maes, W., “Dominant dimer emission provides colour stability for red thermally activated delayed fluorescence emitter” J. Mater. Chem. C 2022, 10, 5840–5848 – DOI: 10.1039/d1tc04913e
Paredis, S.; Cardeynaels, T.; Deckers, J.; Danos, A.; Vanderzande, D.; Monkman, A. P.; Champagne, B.; Maes, W., “Bridge control of photophysical properties in benzothiazole-phenoxazine emitters – from thermally activated delayed fluorescence to room temperature phosphorescence“ J. Mater. Chem. C 2022, 10, 4775–4784 – DOI: 10.1039/D1TC04885F

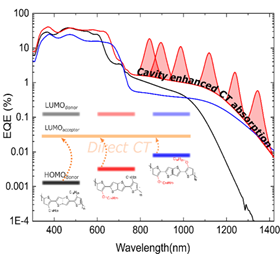
Vanderspikken, J.; Liu, Q.; Liu, Z.; Vandermeeren, T.; Cardeynaels, T.; Gielen, S.; Van Mele, B.; Van den Brande, N.; Champagne, B.; Vandewal, K.; Maes, W., “Tuning electronic and morphological properties for high performance, wavelength-selective organic near-infrared cavity devices” Adv. Funct. Mater. 2021, 31, 2108146 – DOI: 10.1002/adfm.202108146
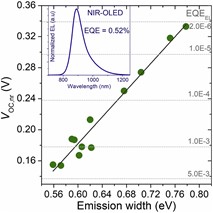
Liu, Q.; Smeets, S.; Mertens, S.; Xia, Y.; Maes, W.; Vandewal, K., “Narrow electroluminescence linewidths for reduced non-radiative recombination in organic photovoltaics and near-infrared light-emitting diodes” Joule 2021, 5, 2365–2379 – DOI: 10.1016/j.joule.2021.06.010
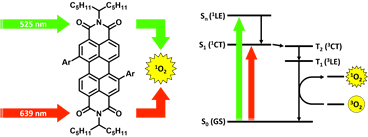
Deckers, J.; Cardeynaels, T.; Champagne, B.; Maes, W., “Heavy-atom-free bay-substituted perylene diimide donor-acceptor photosensitizers” ChemPhysChem 2021, 22, 1488–1496 – DOI: 10.1002/cphc.202100269
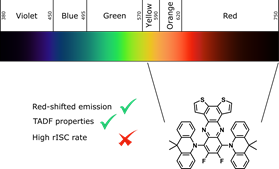
Cardeynaels, T.; Paredis, S.; Danos, A.; Harrison, A.; Deckers, J.; Brebels, S.; Lutsen, L.; Vanderzande, D.; Monkman, A. P.; Champagne, B.; Maes, W., “Difluorodithieno[3,2-a:2',3'-c]phenazine as a strong acceptor for materials displaying TADF or room temperature phosphorescence" Dyes & Pigm. 2021, 190, 109301 – DOI: 10.1016/j.dyepig.2021.109301
Chevrier, M.; Kesters, J.; Houston, J. E.; Van den Brande, N.; Chambon, S.; Richeter, S.; Van Mele, B.; Arnold, T.; Mehdi, A.; Lazzaroni, R.; Dubois, P.; Evans, R. C.; Maes, W.; Clément, S., “Phosphonium-based polythiophene conjugated polyelectrolytes with different surfactant counterions" Polym. Int. 2021, 70, 457–466 – DOI:10.1002/pi.6088
Cardeynaels, T.; Paredis, S.; Danos, A.; Vanderzande, D.; Monkman, A. P.; Champagne, B.; Maes, W., “Benzo[1,2-b:4,5-b']dithiophene as a weak donor component for push-pull materials displaying TADF or room temperature phosphorescence" Dyes & Pigm. 2021, 186, 109022 – DOI: 10.1016/j.dyepig.2020.109022
Gielen, S.; Kaiser, C.; Verstraeten, F.; Kublitski, J.; Benduhn, J.; Spoltore, D.; Verstappen, P.; Maes, W.; Meredith, P.; Armin, A.; Vandewal, K., “Intrinsic Detectivity Limits of Organic Near-Infrared Photodetectors” Adv. Mater. 2020, 2003818 – DOI: 10.1002/adma.202003818
Sudakov, I.; Van Landeghem, M.; Lenaerts, R.; Maes, W.; Van Doorslaer, S.; Goovaerts, E. , “The interplay of stability between donor and acceptor materials in a fullerene-free bulk heterojunction solar cell blend” Adv. Energy Mater. 2020, 2002095 – DOI: 10.1002/aenm.202002095
Beckers, O.; Gielen, S.; Verstraeten, F.; Verstappen, P.; Lutsen, L.; Vandewal, K.; Maes, W., “Continuous droplet flow synthesis of a near-infrared responsive push-pull copolymer toward large scale implementation of organic photodetectors” ACS Appl. Polym. Mater. 2020, 2, 4373–4378 – DOI: 10.1021/acsapm.0c00998
Deckers, J.; Cardeynaels, T.; Penxten, H.; Ethirajan, A.; Ameloot, M.; Kruk, M.; Champagne, B.; Maes, W., “Near-Infrared BODIPY-Acridine Dyads Acting as Heavy-Atom-Free Dual-Functioning Photosensitizers” Chem. Eur. J. 2020, 26, 15212–15225 – DOI:10.1002/chem.202002549
Cardeynaels, T.; Paredis, S.; Deckers, J.; Brebels, S.; Vanderzande, D.; Maes, W.; Champagne, B., “Finding the optimal exchange-correlation functional to describe the excited state properties of push-pull organic dyes designed for TADF" Phys.Chem.Chem.Phys. 2020, 22, 16387–16399 – DOI:10.1039/D0CP02409K
Verstraeten, F.; Gielen, S.; Verstappen, P.; Raymakers, J.; Penxten, H.; Lutsen, L.; Vandewal, K.; Maes, W., “Efficient and readily tuneable near-infrared photodetection up to 1500 nm enabled by thiadiazoloquinoxaline-based push-pull type conjugated polymers” J. Mater. Chem. C 2020, 8, 10098–10103 – DOI: 10.1039/D0TC01435D
Van den Brande, N.; Defour, M.; Devisscher, D.; Verstappen, P.; Reekmans, G.; D’Haen, J.; Adriaensens, P.; Maes, W.; Van Assche, G.; Van Mele, B., “The phase behavior in the active layer of small molecule organic photovoltaics: the state diagram of p-DTS(FBTTh2)2:PC71BM” J. Phys. Chem. C 2020, 124, 7566–7577 – DOI:10.1021/acs.jpcc.0c00808
Raymakers, J.; Artemenko, A.; Verstraeten, F.; Krysova, H.; Cermák, J.; Nicley, S. S.; Lopez-Carballeira, D.; Kromka, A.; Haenen, K.; Kavan, L.; Maes, W.; Rezek, B., “Photogenerated charge collection on diamond electrodes with covalently linked chromophore monolayers” Electrochim Acta 2020, 337, 135762 – DOI:10.1016/j.electacta.2020.135762
Lenaerts, R.; Devisscher, D.; Pirotte, G.; Gielen, S.; Mertens, S.; Cardeynaels, T.; Champagne, B.; Lutsen, L.; Vanderzande, D.; Adriaensens, P.; Verstappen, P.; Vandewal, K.; Maes, W., “The effect of halogenation on PBDTT-TQxT based non-fullerene polymer solar cells – chlorination vs fluorination” Dyes & Pigm. 2020, 181, 108577 – DOI:10.1016/j.dyepig.2020.108577
Fonteyn, P.; Lizin, S.; Maes, W., “The evolution of the most important research topics in organic and perovskite solar cell research from 2008 to 2017:a bibliometric literature review" Sol. Energy Mater. Sol. Cells 2020, 207, 110325 – DOI:10.1016/j.solmat.2019.110325
Genene, Z.; Negash, A.; Abdulahi, B. A.; Thiruvallur Eachambadi, R.; Liu, Z.; Van den Brande, N.; D’Haen, J.; Wang, E.; Vandewal, K.; Maes, W.; Manca, J.; Mammo, W.; Admassie, S., “Comparative Study on the Effect of Alkylsilyl and Alkylthio Side Chains on the Performance of Fullerene and Non-Fullerene Polymer Solar Cells” Org. Electron. 2020, 77, 105572 – DOI:10.1016/j.orgel.2019.105572
Kruk, M. M.; Klenitsky, D. V.; Gladkov, L. L.; Maes, W., “Corrole basicity in the excited states: insights on structure-property relationships” J. Porphyrins Phthalocyanines 2020, 24, 765–774 – DOI:10.1142/S1088424619501797
Ajeeb, Y. H.; Klenitsky, D. V.; Vershilovskaya, I. V.; Petrova, D. V.; Semeikin, A. S.; Maes, W.; Gladkov, L; L.; Kruk, M. M., “Spectral and luminescent properties and NH-tautomerism of alkylated corrole free bases” J. Appl. Spectrosc. 2020, 87, 421–428 – DOI:10.1007/s10812-020-01017-y
Van Landeghem, M.; Lenaerts, R.; Kesters, J.; Maes, W.; Goovaerts, E., “Impact of the donor polymer on recombination via triplet excitons in a fullerene-free organic solar cell” Phys. Chem. Chem. Phys. 2019, 21, 22999–23008 – DOI:10.1039/C9CP03793D
Van den Brande, N.; Defour, M.; Liu, Z.; Verstappen, P.; Nies, E.; Maes, W.; Van Assche, G.; Van Mele, B., “Homocoupling defects of a small donor molecule for organic photovoltaics: quantification of the eutectic state diagram by rapid heat-cool DSC" J. Phys. Chem. C 2019, 123, 22634–22642 – DOI:10.1021/acs.jpcc.9b06336
Raymakers, J.; Haenen, K.; Maes, W., “Diamond Surface Functionalization: From Gemstone to Photoelectrochemical Applications” J. Mater. Chem. C 2019, 7, 10134–10165 – DOI:10.1039/C9TC03381E
Devisscher, D.; Reekmans, G.; Kesters, J.; Verstappen, P.; Benduhn, J.; Van den Brande, N.; Lutsen, L.; Manca, J.; Vanderzande, D.; Vandewal, K.; Adriaensens, P.; Maes, W., “Analysis of Bulk Heterojunction Organic Solar Cell Blends by Solid-State NMR Relaxometry and Sensitive External Quantum Efficiency" Org. Electron. 2019, 74, 309–314 – DOI: 10.1016/j.orgel.2019.06.046
Cheruku, S.; D’Olieslaeger, L.; Smisdom, N.; Smits, J.; Vanderzande, D.; Maes, W.; Ameloot, M.; Ethirajan, A., “Fluorescent PCDTBT nanoparticles with tunable size for versatile bioimaging” Materials 2019, 12, 2497 – DOI:10.3390/ma12152497 – Open Access
Nicley, S. S.; Drijkoningen, S.; Pobedinskas, P.; Raymakers, J.; Maes, W.; Haenen, K., “Growth of boron-doped diamond films on gold-coated substrates with and without gold nanoparticle formation” Cryst. Growth Des. 2019, 19, 3567–3575 – DOI: 10.1021/acs.cgd.9b00488
Kelchtermans, M.; Deckers, J.; Brebels, J.; Kesters, J.; Verstappen, P.; Eachambadi, R. T.; Liu, Z.; Van den Brande, N.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “Homocoupling defects in porphyrinoid small molecules and their effect on organic solar cell performance” Org. Electron. 2019, 69, 48–55 – DOI:10.1016/j.orgel.2019.03.12
Van Landeghem, M.; Kudrjasova, J.; Maes, W.; Goovaerts, E.; Van Doorslaer, S., “EPR characterization of the light-induced negative polaron in a functionalized dithienylthiazolo[5,4-d]thiazole acceptor for organic photovoltaics” Appl. Magn. Reson. 2019, 50, 1253–1265 – DOI: 10.1007/s00723-019-01146-4
Lenaerts, R.; Cardeynaels, T.; Sudakov, I.; Kesters, J.; Verstappen, P.; Manca, J.; Champagne, B.; Lutsen, L.; Vanderzande, D.; Vandewal, K.; Goovaerts, E.; Maes, W., “All-Polymer Solar Cells Based on Photostable Bis(Perylene Diimide) Acceptor Polymers” Sol. Energy Mater. Sol. Cells 2019, 196, 178–184 – DOI:10.1016/j.solmat.2019.03.044
Kruk, M. M.; Klenitsky, D. V.; Maes, W., “Molecular Structure and Conformation of Free Base Corroles” Macroheterocycles 2019, 12, 58–67 – DOI:10.6060/mhc190229k
Braeken, Y.; Cheruku, S.; Seneca, S.; Smisdom, N.; Berden, L.; Kruyfhooft, L.; Penxten, H.; Lutsen, L.; Fron, E.; Vanderzande, D.; Ameloot, M.; Maes, W.; Ethirajan, A., “The effect of branching on the optical properties of poly(p-phenylene ethynylene) conjugated polymer nanoparticles for bioimaging” ACS Biomater. Sci. Eng. 2019, 5, 1967–1977 – DOI:10.1021/acsbiomaterials.8b01416
Negash, A.; Genene, Z.; Thiruvallur Eachambadi, R.; Verstappen, P.; Van den Brande, N.; Kesters, J.; D’Haen, J.; Wang, E.; Vandewal, K.; Maes, W.; Manca, J.; Mammo, W.; Admassie, S., “Ladder-type high gap conjugated polymers based on indacenodithieno[3,2-b]thiophene and bithiazole for organic photovoltaics” Org. Electron. 2019, 74, 211–217 – DOI:10.1016/j.orgel.2019.07.010
Negash, A.; Genene, Z.; Thiruvallur Eachambadi, R.; Kesters, J.; Van den Brande, N.; D’Haen, J.; Penxten, H.; Alikadit, B.; Wang, E.; Vandewal, K.; Maes, W.; Mammo, W.; Manca, J.; Admassie, S., “Diketopyrrolopyrrole-based terpolymers with tunable broad band absorption for fullerene and fullerene-free polymer solar cells” J. Mater. Chem. C 2019, 7, 3375–3384 – DOI:10.1039/C8TC06407E
Chevrier, M.; Lopez, G.; Zajaczkowski, W.; Kesters, J.; Lenaerts, R.; Surin, M.; De Winter, J.; Richeter, S.; Pisula, W.; Mehdi, A.; Gerbaux, P.; Lazzaroni, R.; Dubois, P.; Maes, W.; Ameduri, B.; Clément, S., “Synthesis and properties of a P3HT-based ABA triblock copolymer containing a perfluoropolyether central segment” Synth. Met. 2019, 252, 127–134 – DOI:10.1016/j.synthmet.2019.04.001
Vanpoucke, D. E. P.; Nicley, S. S.; Raymakers, J.; Maes, W.; Haenen, K., “Can Europium atoms form luminescent centres in diamond: a combined theoretical-experimental study” Diam. Relat. Mater. 2019, 94, 233–241 – DOI:10.1016/j.diamond.2019.02.024
Ajeeb, Y. H.; Minchenya, A. A.; Klimovich, P. G.; Maes, W.; Kruk, M. M., “Thermochromism of corrole solutions in ethanol” J. Appl. Spectrosc. 2019, 86, 788–794 – DOI: 10.1007/s10812-019-00894-2
Ajeeb, Y. H.; Karlovich, T. B.; Gladkov, L. L.; Maes, W.; Kruk, M. M., “Temperature Dependence of Excited Singlet S1-State Deactivation in Corrole Free Bases” J. Appl. Spectrosc. 2019, 86, 345–351 (in Russian) / 389–395 (in English) – DOI: 10.1007/s10812-019-00831-3
Brebels, J.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “High Dielectric Constant Conjugated Materials for Organic Photovoltaics”. J. Mater. Chem. A 2017, 5, 24037–24050 (DOI:10.1039/C7TA06808E)
Braeken, Y.; Cheruku, S.; Ethirajan, A.; Maes, W., “Conjugated polymer nanoparticles for bioimaging”. Materials 2017, 10, 1420 (DOI:10.3390/ma10121420) - open access
Knyukshto, V. N.; Ngo, T. H.; Dehaen, W.; Maes, W.; Kruk, M. M., “Phosphorescence of Free Base Corroles”. RSC Adv. 2016, 6, 43911–43915 (DOI:10.1039/C6RA06196F)
Kesters, J.; Verstappen, P.; Kelchtermans, M.; Lutsen, L.; Vanderzande, D.; Maes, W., “Porphyrin-Based Bulk Heterojunction Organic Photovoltaics: The Rise of the Colors of Life”. Adv. Energy Mater. 2015, 1500218 (DOI:10.1002/aenm.201500218)
Preiß, J.; Sachse, T.; Herrmann-Westendorf, F.; Ngo, T. H.; Martínez, T.; Dietzek, B.; Hill, J. P.; Ariga, K.; Kruk, M. M.; Maes, W.; Presselt, M., “Absorption and Fluorescence Features of an Amphiphilic meso-Pyrimidinylcorrole: Experimental Study and Quantum Chemical Calculations”. J. Phys. Chem. A 2017, 121, 8614–8624 (DOI:10.1021/acs.jpca.7b08910)
Verstraeten, F.; Gielen, S.; Verstappen, P.; Kesters, J.; Georgitzikis, E.; Raymakers, J.; Cheyns, D.; Malinowski, P.; Daenen, M.; Lutsen, L.; Vandewal, K.; Maes, W., “Near-infrared organic photodetectors based on bay-annulated indigo showing broadband absorption and high detectivities up to 1.1 µm”. J. Mater. Chem. C 2018, 6, 11645–11650 (DOI:10.1039/C8TC04164D)
Vangerven, T.; Verstappen, P.; Patil, N.; D’Haen, J.; Cardinaletti, I.; Benduhn, J.; Van den Brande, N.; Defour, M.; Lemaur, V.; Beljonne, D.; Lazzaroni, R.; Champagne, B.; Vandewal, K.; Andreasen, J. W.; Adriaensens, P.; Breiby, D. B.; Van Mele, B.; Vanderzande, D.; Maes, W.; Manca, J., “Elucidating Batch-to-batch Variation Caused by Homocoupled Side Products in Solution Processable Organic Solar Cells“. Chem. Mater. 2016, 28, 9088–9098 (DOI:10.1021/acs.chemmater.6b04143)
Vangerven, T.; Verstappen, P.; Drijkoningen, J.; Dierckx, W.; Himmelberger, S.; Salleo, A.; Vanderzande, D.; Maes, W.; Manca, J. V., “Molar mass versus polymer solar cell performance: Highlighting the role of homocouplings”. Chem. Mater. 2015, 27, 3726–3732 (DOI:10.1021/acs.chemmater.5b00939)
Vershilovskaya, I. V.; Stefani, S.; Verstappen, P.; Ngo, T. H.; Scheblykin, I. G.; Dehaen, W.; Maes, W.; Kruk, M. M., “Spectral-Luminescent Properties of Meso-Tetraarylporphyrins Revisited: the Role of Aryl Type, Substitution Pattern and Macrocycle Core Protonation”. Macroheterocycles 2017, 10, 257–267 (DOI:10.6060/mhc160962n)
Raymakers, J.; Krysova, H.; Artemenko, A.; Čermák, J.; Nicley, S. S.; Verstappen, P.; Gielen, S.; Kromka, A.; Haenen, K.; Kavan, L.; Maes, W.; Rezek, B., “Functionalization of Boron-Doped Diamond with a Push-Pull Chromophore via Sonogashira and CuAAC Chemistry”. RSC Adv. 2018, 8, 33276–33290 (DOI:10.1039/c8ra07545j) - open access
Defour, M.; Van den Brande, N.; Van Lokeren, L.; Van Assche, G.; Maes, W.; Vanderzande, D.; Van Mele, B., “Influence of the amorphous phase and preceding solution processing on the eutectic behaviour in the state diagram of P3HT:PC61BM determined by rapid. RSC Adv. 2016, 6, 92981–92988 (DOI:10.1039/C6RA20659J)
Beenken, W.; Maes, W.; Kruk, M.; Martinez, T.; Presselt, M., “Origin of the individual basicity of corrole NH-tautomers: a quantum chemical study on molecular structure and dynamics, kinetics, and thermodynamics. J. Phys. Chem. A 2015, 119, 6875–6883 (DOI:10.1021/acs.jpca.5b02869)
Raymakers, J.; Artemenko, A.; Nicley, S. S.; Štenclová, P.; Kromka, A.; Haenen, K.; Maes, W.; Rezek, B., “Expanding the scope of diamond surface chemistry: Stille and Sonogashira cross-coupling reactions”. J. Phys. Chem. C 2017, 121, 23446–23454 (DOI:10.1021/acs.jpcc.7b06426)
Pirotte, G.; Kesters, J.; Cardeynaels, T.; Verstappen, P.; D’Haen, J.; Lutsen, L.; Champagne, B.; Vanderzande, D.; Maes, W., “The impact of acceptor-acceptor homocoupling on the optoelectronic properties and photovoltaic performance of PDTSQxff low bandgap polymers”. Macromol. Rapid Commun. 2018, 39, 1800086 (DOI:10.1002/marc.201800086)
Thomas, J.; Dobrzańska, L.; Van Meervelt, L.; Quevedo, M. A.; Woźniak, K.; Stachowicz, M.; Smet, M.; Maes, W.; Dehaen, W., “Homodiselenacalix[4]arenes: molecules with unique channeled crystal structures”. Chem. Eur. J. 2016, 22, 979–987 (DOI:10.1002/chem.201503385)
Pirotte, G.; Kesters, J.; Verstappen, P.; Govaerts, S.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “Continuous Flow Polymer Synthesis toward Reproducible Large-Scale Production for Efficient Bulk Heterojunction Organic Solar Cells”. ChemSusChem 2015, 8, 3228–3233 (DOI:10.1002/cssc.201500850)
Pirotte, G.; Agarkar, S.; Xu, B.; Zhang, J.; Lutsen, L.; Vanderzande, D.; Yan, H.; Pollet, P.; Reynolds, J. R.; Maes, W.; Marder, S. R., “Molecular weight tuning of low bandgap polymers by continuous flow chemistry: increasing the applicability of PffBT4T for organic photovoltaics”. J. Mater. Chem. A 2017, 5, 18166–18175 (DOI: 10.1039/C7TA05627C)
Cardinaletti, I.; Vangerven, T.; Nagels, S.; Cornelissen, R.; Schreurs, D.; Hruby, J.; Vodnik, J.; Devisscher, D.; Kesters, J.; D’Haen, J.; Franquet, A.; Spampinato, V.; Conard, T.; Maes, W.; Deferme, W.; Manca, J. V., "Organic and perovskite solar cells for space applications". Sol. Energy Mater. Sol. Cells 2018, 182, 121–127 (DOI:10.1016/j.solmat.2018.03.24)
Heckler, I. M.; Kesters, J.; Defour, M.; Penxten, H.; Van Mele, B.; Maes, W.; Bundgaard, E., “A stability study of polymer solar cells using conjugated polymers with different donor or acceptor side chain patterns”. J. Mater. Chem. A 2016, 4, 16677–16689 (DOI:10.1039/C6TA07244E)
Krysova, H.; Kavan, L.; Vlcková-Zivcová, Z.; Yeap, W. S.; Verstappen, P.; Maes, W.; Haenen, K.; Gao, F.; Nebel, C. E., “Dye-Sensitization of Boron-Doped Diamond Foam: Champion Photoelectrochemical Performance of Diamond Electrodes under Solar Light Illumination”. RSC Adv. 2015, 5, 81069–81077 (DOI:10.1039/C5RA12413A)
Govaerts, S.; Kesters, J.; Defour, M.; Van Mele, B.; Penxten, H.; Neupane, S.; Renner, F. U.; Lutsen, L.; Vanderzande, D.; Maes, W., “Conjugated ionic (co)polythiophene-based cathode interlayers for bulk heterojunction organic solar cells”. Eur. Polym. J. 2017, 97, 49–56 (DOI:10.1016/j.eurpolymj.2017.09.043)
Di Carlo Rasi, D.; Hendriks, K. H.; Simone, G.; Gelinck, G. H.; Gevaerts, V. S.; Andriessen, R.; Pirotte, G.; Maes, W.; Li, W.; Wienk, M. M.; Janssen, R. A. J., “A universal route to fabricate n-i-p multi-junction polymer solar cells via solution processing”. Solar RRL 2018, 2, 1800018 (DOI:10.1002/solr.201800018)
Govaerts, S.; Verstappen, P.; Penxten, H.; Defour, M.; Van Mele, B.; Lutsen, L.; Vanderzande, D.; Maes, W., “Synthesis of highly fluorescent all-conjugated alternating donor-acceptor (block) copolymers via GRIM polymerization”. Macromolecules 2016, 49, 6411–6419 (DOI:10.1021/acs.macromol.6b01389)
Verstappen, P.; Kesters, J.; D’Olieslaeger, L.; Drijkoningen, J.; Cardinaletti, I.; Vangerven, T.; Bruijnaers, B. J.; Willems, R. E. M.; D’Haen, J.; Manca, J. V.; Lutsen, L.; Vanderzande, D. J. M.; Maes, W., “Simultaneous enhancement of solar cell efficiency and stability by reducing the side chain density on fluorinated PCPDTQx copolymers”. Macromolecules 2015, 48, 3873–3882 (DOI:10.1021/acs.macromol.5b00035)
Brebels, J.; Klider, K. C. C. W. S.; Kelchtermans, M.; Verstappen, P.; Van Landeghem, M.; Van Doorslaer, S.; Goovaerts, E.; Garcia, J; R.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “Low bandgap polymers based on bay-annulated indigo for organic photovoltaics: enhanced sustainability in material design and solar cell fabrication“. Org. Electron. 2017, 50, 264–272 (DOI:10.1016/j.orgel.2017.07.037)
Van Landeghem, M.; Maes, W.; Goovaerts, E.; Van Doorslaer, S., “Disentangling overlapping high-field EPR spectra of organic radicals: identification of light-induced polarons in record fullerene-free solar cells". J. Magn. Reson. 2018, 288, 1–10 (DOI:10.1016/j.jmr.2018.01.007)
Verstappen, P.; Cardinaletti, I.; Vangerven, T.; Vanormelingen, W.; Verstraeten, F.; Lutsen, L.; Vanderzande, D.; Manca, J.; Maes, W., “Impact of structure and homo-coupling of the central donor unit of small molecule organic semiconductors on solar cell performance”. RSC Adv. 2016, 6, 32298–32307 (DOI:10.1039/C6RA06146J) - open access
Marin, L.; Kudrjasova, J.; Verstappen, P.; Penxten, H.; Robeyns, K.; Lutsen, L.; Vanderzande, D.; Maes, W., “Quinoxaline-based cyclo(oligophenylenes)”. J. Org. Chem. 2015, 80, 2425–2430 (DOI:10.1021/jo502771a)
D’Olieslaeger, L.; Braeken, Y.; Cheruku, S.; Smits, J.; Ameloot, M.; Vanderzande, D.; Maes, W.; Ethirajan, A., “Tuning the optical properties of poly(p-phenylene ethynylene) nanoparticles as bio-imaging probes by side chain functionalization“. J. Colloid Interface Sci. 2017, 504, 527–537 (DOI:10.1016/j.jcis.2017.05.072)
Pirotte, G.; Verstappen, P.; Vanderzande, D.; Maes, W., “On the ‘true’ structure of push-pull type low bandgap polymers for organic electronics”. Adv. Electron. Mater. 2018, 1700481 (DOI:10.1002/aelm.201700481)
Maes, V.; Pirotte, G.; Brebels, J.; Verstappen, P.; Lutsen, L.; Vanderzande, D.; Maes, W., “Synthesis of N,N’-dialkyl-6,6’-dibromoisoindigo Derivatives by Continuous Flow”. J. Flow. Chem. 2015, 5, 201–209 (DOI:10.1556/1846.2015.00033)
D’Olieslaeger, L.; Pirotte, G.; Cardinaletti, I.; D’Haen, J.; Manca, J.; Vanderzande, D.; Maes, W.; Ethirajan, A., “Eco-Friendly Fabrication of PBDTTPD:PC71BM Solar Cells Reaching a PCE of 3.8% Using Water-Based Nanoparticle Dispersions”. Org. Electron. 2017, 42, 42–46 (DOI:10.1016/j.orgel.2016.12.018)
Stoltsfuz, D. M.; Kesters, J.; Kelchtermans, M.; Verstappen, P.; Cardinaletti, I.; Cornelissen, R.; D’Haen, J.; Lutsen, L.; Vanderzande, D.; Manca, J.; Bielawski, C. W.; Maes, W.; Sessler, J. L., “Improved Efficiency of Polymer-Fullerene Bulk Heterojunction Solar Cells by the Addition of Cu(II)-Porphyrin-Oligothiophene Conjugates”. Synth. Metals 2016, 218, 1–8 (DOI:10.1016/j.synthmet.2016.04.026)
Brebels, J.; Douvogianni, J.; Devisscher, D.; Eachambadi, R. T.; Manca, J.; Lutsen, L.; Vanderzande, D.; Hummelen, J. C.; Maes, W., “An Effective Strategy to Enhance the Dielectric Constant of Organic Semiconductors – CPDTTPD-Based Low Bandgap Polymers Bearing OEG Side Chains". J. Mater. Chem. C 2018, 6, 500–511 (DOI:10.1039/C7TC05264B)
D’Olieslaeger, L.; Pfannmöller, M.; Fron, E.; Cardinaletti, I.; Van Der Auweraer, M.; Van Tendeloo, G.; Bals, S.; Maes, W.; Vanderzande, D.; Manca, J.; Ethirajan, A., “Tuning of PCDTBT:PC71BM blend nanoparticles for eco-friendly processing of polymer solar cells”. Sol. Energy Mater. Sol. Cells 2017, 159, 179–188 (DOI:10.1016/j.solmat.2016.09.008)
Heckler, I.; Kesters, J.; Defour, M.; Madsen, M. V.; Penxten, H.; D’Haen, J.; Van Mele, B.; Maes, W.; Bundgaard, E., “The influence of conjugated polymer side chain manipulation on the efficiency and stability of polymer solar cells”. Materials 2016, 9, 181 (DOI:10.3390/ma9030181) - open access
Chevrier, M.; Houston, J. E.; Kesters, J.; Van den Brande, N.; Terry, A. E.; Richeter, S.; Mehdi, A.; Coulembier, O.; Dubois, P.; Lazzaroni, R.; Van Mele, B.; Maes, W.; Evans, R. E.; Clément, S., “Self-Assembled Conjugated Polyelectrolyte-Surfactant Complexes as Efficient Cathode Interlayer Materials for Bulk Heterojunction Organic Solar Cells”. J. Mater. Chem. A 2015, 3, 23905–23916 (DOI:10.1039/C5TA06966A)
Brebels, J.; Kesters, J.; Defour, M.; Pirotte, G.; Van Mele, B.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “A PCPDTTPD-based narrow bandgap conjugated polyelectrolyte for organic solar cells”. Polymer 2018, 137, 303–311 (DOI:10.1016/j.polymer.2018.01.027)
Marchal, W.; Verboven, I.; Kesters, J.; Moeremans, B.; De Dobbelaere, C.; Bonneux, G.; Elen, K.; Conings, B.; Maes, W.; Boyen, H.-G.; Deferme, W.; Van Bael, M. K.; Hardy, A., “Steering the performance of MoO3 hole transporting layers for organic photovoltaics: interface morphology vs. electronic structure”. Materials 2017, 10, 123 (DOI:10.3390/ma10020123) - open access
Kesters, J.; Govaerts, S.; Pirotte, G.; Drijkoningen, J.; Chevrier, M.; Van den Brande, N.; Liu, X.; Fahlman, M.; Van Mele, B.; Lutsen, L.; Vanderzande, D.; Manca, J.; Clément, S.; Von Hauff, E.; Maes, W., “High Permittivity Conjugated Polyelectrolyte Interlayers for High Performance Bulk Heterojunction Organic Solar Cells”. ACS Appl. Mater. Interfaces 2016, 8, 6309–6314 (DOI:10.1021/acsami.6b00242) - open access
Marchal, W.; De Dobbelaere, C.; Kesters, J.; Bonneux, G.; Vandenbergh, J.; Damm, H.; Junkers, T.; Maes, W.; D’Haen, J.; Van Bael, M.; Hardy, A., “Combustion deposition of MoO3 thin films: from fundamentals to OPV applications”. RSC Adv. 2015, 5, 91349–91362 (DOI:10.1039/C5RA18001E)
Perthué, A.; Fraga Domínguez, I.; Verstappen, P.; Maes, W.; Dautel, O. J.; Wantz, G.; Rivaton, A., “An efficient and simple tool for assessing singlet oxygen involvement in the photo-oxidation of conjugated materials”. Sol. Energy Mater. Sol. Cells 2018, 176, 336–339 (DOI:10.1016/j.solmat.2017.10.019)
Kudrjasova, J.; Van Landeghem, M.; Vangerven, T.; Kesters, J.; Heintges, G. H. L.; Cardinaletti, I.; Lenaerts R.; Penxten, H.; Adriaensens, P.; Lutsen, L.; Vanderzande, D.; Manca, J.; Goovaerts, E.; Maes, W., “Designing Small Molecule Organic Solar Cells with High Open-Circuit Voltage”. ChemistrySelect 2017, 2, 1253–1261 (DOI:10.1002/slct.201601915)
Chevrier, M.; Kesters, J.; Blayo, C.; Richeter, S.; Van Der Lee, A.; Coulembier, O.; Surin, M.; Mehdi, A.; Lazzaroni, R.; Evans, R. C.; Maes, W.; Dubois, P.; Clément, S., “Regioregular Polythiophene-Porphyrin Supramolecular Copolymers for Optoelectronic Applications”. Macromol. Chem. Phys. 2016, 217, 445–458 (DOI:10.1002/macp.201500280)
Tomassetti, M.; Ouhib, F.; Cardinaletti, I.; Verstappen, P.; Salleo, A.; Jérôme, C.; Manca, J.; Maes, W.; Detrembleur, C., “Branched and linear A2-D-A1-D-A2 isoindigo-based solution-processable small molecules for organic field-effect transistors and solar cells”. RSC Adv. 2015, 5, 85460–85469 (DOI:10.1039/C5RA17660C)
Kudrjasova, J.; Kesters, J.; Verstappen, P.; Brebels, J.; Vangerven, T.; Cardinaletti, I.; Drijkoningen, J.; Penxten, H.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “A direct arylation approach towards efficient small molecule organic solar cells”. J. Mater. Chem. A 2016, 4, 791–795 (DOI:10.1039/C5TA09023G) - open access
Braeken, Y.; Verstappen, P.; Lutsen, L.; Vanderzande, D.; Maes, W., “Synthesis of a multifunctional poly(p-phenylene ethylene) scaffold with clickable azide-containing side chains”. Polym. Chem. 2015, 6, 6720–6731 (DOI:10.1039/C5PY00741K)
Tomassetti, M.; Ouhib, F.; Wislez, A.; Duwez, A.-S.; Penxten, H.; Dierckx, W.; Bovee, R.; van Pruissen, G. W. P.; Jérôme, C.; Manca, J.; Maes, W.; Detrembleur, C., “Low bandgap copolymers based on monofluorinated isoindigo toward efficient polymer solar cells”. Polym. Chem. 2015, 6, 6040–6049 (DOI:10.1039/C5PY00693G)
Presselt, M.; Dehaen, W.; Maes, W.; Klamt, A.; Martinez, T.; Beenken, W. J. D.; Kruk, M. M., “Quantum Chemical Insights into the Dependence of Porphyrin Basicity on the meso-Aryl Substituents: Thermodynamics, Buckling, Reaction Sites and Molec. Phys. Chem. Chem. Phys. 2015, 17, 14096–14106 (DOI:10.1039/C5CP01808K)
Marín V., A.; Aguirre, M. J.; Muena, J. P.; Dehaen, W.; Maes, W.; Ngo, T. H.; Ramírez, G.; Arévalo, M. C., “Electro-reduction of Oxygen to Water Mediated by Stable Glassy Carbon Electrodes Modified by Co(II)-porphyrins with Voluminous Meso-Substituents”. Int. J. Electrochem. Sci. 2015, 10, 3949–3960 - open access
Spoltore, D.; Vangerven, T.; Verstappen, P.; Piersimoni, F.; Bertho, S.; Vandewal, K.; Van den Brande, N.; Defour, M.; Van Mele, B.; De Sio, A.; Parisi, J.; Lutsen, L.; Vanderzande, D.; Maes, W.; Manca, J. V., “Effect of molecular weight on morphology and photovoltaic properties in P3HT:PCBM solar cells”. Org. Electron. 2015, 21, 160–170 (DOI:10.1016/j.orgel.2015.02.017)
Kesters, J.; Verstappen, P.; Raymakers, J.; Vanormelingen, W.; Drijkoningen, J.; D’Haen, J.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “Enhanced Organic Solar Cell Stability by Polymer (PCPDTBT) Side Chain Functionalization”. Chem. Mater. 2015, 27, 1332–1341 (DOI:10.1021/cm504391k)
Ouhib, F.; Tomassetti, M.; Dierckx, W.; Verstappen, P.; Wisley, A.; Duwez, A.-S.; Lemaur, V.; Lazzaroni, R.; Manca, J.; Maes, W.; Jérôme, C.; Detrembleur, C., “Linear and propeller-like fluoro-isoindigo based donor-acceptor small molecules for organic solar cells”. Org. Electron. 2015, 20, 76–88 (DOI:10.1016/j.orgel.2015.01.033)
Gilissen, K.; Stryckers, J.; Drijkoningen, J.; Heintges, G. H. L.; Verstappen, P.; Lutsen, L.; Manca, J.; Maes, W.; Deferme, W., “Ultrasonic spray coating as deposition technique for the light-emitting layer in Polymer LEDs”. Org. Electron. 2015, 20, 31–35 (DOI:10.1016/j.orgel.2015.01.015)
Dierckx, W.; Oosterbaan, W. D.; Bolsée, J.-C.; Cardinaletti, I.; Maes, W.; Boyen, H.-G.; D’Haen, J.; Nesladek, M.; Manca, J., “Organic phototransistors using poly(3-hexylthiophene) nanofibres”. Nanotechnology 2015, 26, 065201 (DOI:10.1088/0957-4484/26/6/065201)
Kesters, J.; Verstappen, P.; Vanormelingen, W.; Drijkoningen, J.; Vangerven, T.; Devisscher, D.; Marin, L.; Champagne, B.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “N-Acyl-dithieno[3,2-b:2’,3’-d]pyrrole based low bandgap copolymers affording improved open-circuit voltages and efficiencies in polymer solar cells”. Sol. Energy Mater. Sol. Cells 2015, 136, 70–77 (DOI:10.1016/j.solmat.2014.12.037)
Verstappen, P.; Kesters, J.; Vanormelingen, W.; Heintges, G.; Drijkoningen, J.; Vangerven, T.; Marin, L.; Koudjina, S.; Champagne, B.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., “Fluorination as an effective tool to increase the open-circuit voltage and charge carrier mobility of organic solar cells based on poly(cyclopenta[2,. J. Mater. Chem. A 2015, 3, 2960–2970 (DOI:10.1039/C4TA06054G)
93. ”Worldwide outdoor round robin study of organic photovoltaic devices and modules”: Madsen, M.; Gevorgyan, S. A.; Pacios, R.; Ajuria, J.; Etxebarria, I.; Kettle, J. P.; Bristow, N. D.; Neophytou, M.; Choulis, S. A.; Roman, L. S.; Yohannes, T.; Cester, A.; Cheng, P.; Zhan, X.; Wu, J.; Xie, Z.; Tu, W.-C.; He, J.-H.; Fell, C. J.; Anderson, K.; Hermenau, M.; Bartesaghi, D.; Koster, L. J. A.; Machui, F.; González-Valls, I.; Lira-Cantu, M.; Khlyabich, P. P.; Thompson, B. C.; Gupta, R.; Shanmugam, K.; Kulkarni, G. U.; Galagan, Y.; Urbina, A.; Abad, J.; Roesch, R.; Hoppe, H.; Morvillo, P.; Bobeico, E.; Panaitescu, E.; Menon, L.; Luo, Q.; Wu, Z.; Ma, C.; Hambarian, A.; Melikyan, V.; Hambsch, M.; Burn, P. L.; Meredith, P.; Rath, T.; Dunst, S.; Trimmel, G.; Bardizza, G.; Müllejans, H.; Goryachev, A. E.; Misra, R. K.; Katz, E. A.; Takagi, K.; Magaino, S.; Saito, H.; Aoki, D.; Sommeling, P. M.; Kroon, J. M.; Vangerven, T.; Manca, J.; Kesters, J.; Maes, W.; Bobkova, O. D.; Trukhanov, V. A.; Paraschuk, D. Y.; Castro, F. A.; Blakesley, J.; Tuladhar, S. M.; Röhr, J. A.; Nelson, J.; Xia, J.; Parlak, E. J.; Tumay, T. A.; Egelhaaf, H.-J.; Tanenbaum, D. M.; Ferguson, G. M.; Carpenter, R.; Chen, H.; Zimmermann, B.; Hirsch, L.; Wantz, G.; Sun, Z.; Singh, P.; Bapat, C.; Offermans, T.; Krebs, F. C. Sol. Energy Mater. Sol. Cells 2014, 130, 281–290 (IF2014 5.337) – DOI:10.1016/j.solmat.2014.07.021
92. “Diamond functionalization with light-harvesting molecular wires: improved surface coverage by optimized Suzuki cross-coupling conditions”: Yeap, W. S.; Bevk, D.; Liu, X. J.; Krysova, H.; Pasquarelli, A.; Vanderzande, D.; Lutsen, L.; Kavan, L.; Fahlman, M.; Maes, W.; Haenen, K., RSC Adv. 2014, 4, 42044–42053 (IF2014 3.840) – DOI:10.1039/C4RA04740K - Open Access
91. “Functionalization of boron-doped nanocrystalline diamond with N3 dye molecules”: Yeap, W. S.; Liu, X. J.; Bevk, D.; Lutsen, L.; Fahlman, M.; Maes, W.; Haenen, K., ACS Appl. Mater. Interfaces 2014, 6, 10322–10329 (IF2014 6.723) – DOI:10.1021/am501783b
90. “Boron-Doped Diamond Functionalization by an Electrografting - Alkyne-Azide Click Chemistry Sequence”: Yeap, W. S.; Murib, M. S.; Cuypers, W.; Liu, X. J.; van Grinsven, B.; Ameloot, M.; Fahlman, M.; Wagner, P.; Maes, W.; Haenen, K., ChemElectroChem 2014, 1, 1145–1154 (IF2015 3.506) – DOI:10.1002/celc.201402068
89. “Toward bulk heterojunction organic solar cells with stable active layer morphology”: Cardinaletti, I.; Kesters, J.; Bertho, S.; Conings, B.; Piersimoni, F.; D’Haen, J.; Lutsen, L.; Nesladek, M.; Van Mele, B.; Van Assche, G.; Vandewal, K.; Salleo, A.; Vanderzande, D.; Maes, W.; Manca, J. V., J. Photon. Energy 2014, 4, 040997-1–040997-12 (IF2014 1.366) – DOI:10.1117/1.JPE.4.040997
88. “Direct arylation as a versatile tool towards thiazolo[5,4-d]thiazole-based semiconducting materials”: Kudrjasova, J.; Herckens, R.; Penxten, H.; Adriaensens, P.; Lutsen, L.; Vanderzande, D.; Maes, W. Org. Biomol. Chem. 2014, 12, 4663–4672 (IF2014 3.562) – DOI:10.1039/C4OB00360H
87. “Poly(3-alkylthiophene) nanofibers for optoelectronic devices”: Dierckx, W.; Oosterbaan, W. D.; Bolsée, J.-C.; Maes, W.; Vanderzande, D.; Manca, J. J. Mater. Chem. C 2014, 2, 5730–5746 (IF2014 4.696) – DOI:10.1039/C4TC00308J
86. “Investigating the role of efficiency enhancing interlayers for bulk heterojunction solar cells by scanning probe microscopy”: Drijkoningen, J.; Kesters, J.; Vangerven, T.; Lutsen, L.; Vanderzande, D.; Maes, W.; D’Haen, J.; Manca, J., Org. Electron. 2014, 15, 1282–1289 (IF2014 3.827) – DOI:10.1016/j.orgel.2014.02.025
85. “Enhanced open-circuit voltage in polymer solar cells by dithieno[3,2-b:2’,3’-d]pyrrole N-acylation”: Vanormelingen, W.; Kesters, J.; Verstappen, P.; Drijkoningen, J.; Kudrjasova, J.; Koudjina, S.; Liégeois, V.; Champagne, B.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W., J. Mater. Chem. A 2014, 2, 7535–7545 (IF2014 7.443) – DOI:10.1039/C4TA00525B
84. “Amphiphilic N-methylimidazole-functionalized diblock copolythiophenes for organic photovoltaics”: Ghoos, T.; Van Den Brande, N.; Defour, M.; Brassinne, J.; Fustin, C.-A.; Gohy, J.-F.; Hoeppener, S.; Schubert, U. S.; Vanormelingen, W.; Lutsen, L.; Vanderzande, D. J.; Van Mele, B.; Maes, W. Eur. Polym. J. 2014, 53, 206–214 (IF2014 3.005) – DOI:10.1016/j.eurpolymj.2014.01.028
83. “Synthetic protocols toward homodithiacalix[n]arenes”: Thomas, J.; Dobrzanska, L.; Sonawane, M. P.; Smet, M.; Maes, W.; Dehaen, W. Supramol. Chem. 2014, 26, 591–596 (IF2014 2.394) – DOI:10.1080/10610278.2013.875173
82. “Molecular structures and absorption spectra assignment of corrole NH tautomers”: Beenken, W. J. D.; Presselt, M.; Ngo, T. H.; Dehaen, W.; Maes, W.; Kruk, M. M. J. Phys. Chem. A 2014, 118, 862–871 (IF2014 2.693) – DOI:10.1021/jp411033h
81. “Electronic structure of positive and negative polarons in functionalized dithienylthiazolo[5,4-d]thiazoles: a combined EPR and DFT study”: Ling, Y.; Van Mierloo, S.; Schnegg, A.; Fehr, M.; Adriaensens, P.; Lutsen, L.; Vanderzande, D.; Maes, W.; Goovaerts, E.; Van Doorslaer, S. Phys. Chem. Chem. Phys. 2014, 16, 10032–10040 (IF2014 4.493) – DOI:10.1039/C3CP54635G
80. “Synthesis of ester side-chain functionalized diblock copolythiophenes via the Rieke method”: Kudret, S.; Van den Brande, N.; Defour, M.; Van Mele, B.; Lutsen, L.; Vanderzande, D.; Maes, W. Polym. Chem. 2014, 5, 1832–1837 (IF2014 5.520) – DOI:10.1039/C3PY01661G
79. “Enhanced intrinsic stability of the bulk heterojunction active layer blend of polymer solar cells by varying the polymer side chain pattern”: Kesters, J.; Kudret, S.; Bertho, S.; Van den Brande N.; Defour, M.; Van Mele B.; Penxten, H.; Lutsen, L.; Manca, J.; Vanderzande, D.; Maes, W. Org. Electron. 2014, 15, 549–562 (IF2014 3.827) – DOI:10.1016/j.orgel.2013.12.006
78. “Facile synthesis of 3-(ω-acetoxyalkyl)thiophenes and derived copolythiophenes using Rieke zinc”: Kudret, S.; Kesters, J.; Janssen, S.; Van den Brande, N.; Defour, M.; Van Mele, B.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W. React. Funct. Polym. 2014, 75, 22–30 (IF2014 2.515) – DOI:10.1016/j.reactfunctpolym.2013.11.009
77. “Multiple Redox-Active Sites in Copper Dipyrromethene–Corrole Self-Assembled Monolayers Deposited onto Gold Electrodes”: Grabowska, I.; Maes, W.; Ngo, T. H.; Rohand, T.; Dehaen, W.; Radecka, H.; Radecki, J. Int. J. Electrochem. Sci. 2014, 9, 1232–1249 (IF2014 1.500) - Open Access
76. “Solution-processed bi-layer polythiophene-fullerene organic solar cells”: Ghoos, T.; Malinkiewicz, O.; Conings, B.; Manca, J.; Lutsen, L.; Vanderzande, D. J.; Bolink, H. J.; Maes, W. RSC Adv. 2013, 3, 25197–25203 (IF2013 3.708) – DOI:10.1039/C3RA43986K
75. “Imidazolium-substituted ionic (co)polythiophenes: compositional influence on solution behavior and thermal properties”: Ghoos, T.; Brassinne, J.; Fustin, C.-A.; Gohy, J.-F.; Defour, M.; Van den Brande, N.; Van Mele, B.; Lutsen, L.; Vanderzande, D. J.; Maes, W. Polymer 2013, 54, 6293–6304 (IF2013 3.766) – DOI:10.1016/j.polymer.2013.09.049
74. “In Situ Monitoring the Thermal Degradation of PCPDTBT Low Band Gap Polymers with Varying Alkyl Side-Chain Patterns”: Marin, L.; Penxten, H.; Van Mierloo, S.; Carleer, R.; Lutsen, L.; Vanderzande, D.; Maes, W. J. Polym. Sci. A: Polym. Chem. 2013, 51, 4912–4922 (IF2013 3.245) – DOI:10.1002/pola.26920
73. “Life cycle analyses of organic photovoltaics: a review”: Lizin, S.; Van Passel, S.; De Schepper, E.; Maes, W.; Lutsen, L.; Manca, J.; Vanderzande, D. Energy Environ. Sci. 2013, 6, 3136–3149 (IF2013 15.490) – DOI:10.1039/C3EE42653J - Open Access
72. “Synthetic routes toward asymmetrically substituted (functionalized) 4H-cyclopenta[2,1-b:3,4-b']dithiophenes”: Vanormelingen, W.; Verstappen, P.; Maes, V.; Bevk, D.; Lutsen, L.; Vanderzande, D.; Maes, W. Synlett 2013, 24, 2389–2392 (IF2013 2.463) – DOI:10.1055/s-0033-1339707
71. “Actuated conformational switching in a homodithiacalix[4]arene single crystal”: Thomas, J.; Reekmans, G.; Adriaensens, P.; Van Meervelt, L.; Smet, M.; Maes, W.; Dobrzańska, L.; Dehaen, W. Angew. Chem. Int. Ed. 2013, 52, 10237–10240 (IF2013 11.336) – DOI:10.1002/anie.201302822
70. “Quinoxaline derivatives with broadened absorption patterns”: Marin, L.; Lutsen, L.; Vanderzande, D.; Maes, W. Org. Biomol. Chem. 2013, 11, 5866–5876 (IF2013 3.487) – DOI:10.1039/C3OB41059E
69. “Influence of fullerene photodimerization on the PCBM crystallization in polymer:fullerene bulk heterojunctions under thermal stress”: Piersimoni, F.; Degutis, G.; Bertho, S.; Vandewal, K.; Spoltore, D.; Vangerven, T.; Drijkoningen, J.; Van Bael, M. K.; Hardy, A.; D’Haen, J.; Maes, W.; Vanderzande, D.; Nesladek, M.; Manca, J. J. Polym. Sci. B: Polym. Phys. 2013, 51, 1209–1214 (IF2013 2.548) – DOI:10.1002/polb.23330
68. “Improving Efficiencies by Modulating the Central Metal Ion in Porphyrin-Oligothiophene-mediated P3HT/PCBM Organic Solar Cells”: Lyons, D. M.; Kesters, J.; Maes, W.; Bielawski, C. W.; Sessler, J. L. Synth. Metals 2013, 178, 56–61 (IF2013 2.222) – DOI:10.1016/j.synthmet.2013.06.013
67. “Thiazolo[5,4-d]thiazoles – increasingly popular building blocks in material chemistry”: Bevk, D.; Marin, L.; Lutsen, L.; Vanderzande, D.; Maes, W. RSC Adv. 2013, 3, 11418–11431 (IF2013 3.708) – DOI:10.1039/C3RA40851E
66. “Imidazolium-substituted polythiophenes as efficient electron transport materials improving photovoltaic performance”: Kesters, J.; Ghoos, T.; Penxten, H.; Drijkoningen, J.; Vangerven, T.; Lyons, D. M.; Verreet, B.; Aernouts, T.; Lutsen, L.; Vanderzande, D.; Manca, J.; Maes, W. Adv. Energy Mater. 2013, 3, 1180–1185 (IF2013 14.385) – DOI:10.1002/aenm.201300049
65. “Direct 4-electron reduction of molecular oxygen to water mediated by a Cu-10-(4-aminophenyl)-5,15-dimesitylcorrole-modified electrode”: Isaacs, F.; Dehaen, W.; Maes, W.; Ngo, T. H.; Ruiz-León, D.; Herrera, F.; Arce, R.; Arévalo, C.; Aguirre, M. J. Int. J. Electrochem. Sci. 2013, 8, 3406–3418 (IF2013 1.956) - Open Access
64. “An Efficient and Reproducible Procedure for the Preparation of Highly Reactive Rieke Zinc (Zn*)”: Kudret, S.; Oosterbaan, W.; D’Haen, J.; Lutsen, L.; Vanderzande, D.; Maes, W. Adv. Synth. Catal. 2013, 355, 569–575 (IF2013 5.542) – DOI:10.1002/adsc.201201077
63. “Reaction of 4H-cyclopenta[2,1-b:3,4-b']dithiophenes with NBS - a route toward 2H-cyclopenta[2,1-b:3,4-b']dithiophene-2,6(4H)-diones”: Marin, L.; Van Mierloo, S.; Zhang, Y.; Robeyns, K.; Champagne, B.; Adriaensens, P.; Lutsen, L.; Vanderzande, D.; Maes, W. Tetrahedron 2013, 69, 2260–2267 (IF2013 2.817) – DOI:10.1016/j.tet.2013.01.026
62. “Trivalent organophosphorus reagent induced pinacol rearrangement of 4H-cyclopenta[2,1-b:3,4-b']dithiophen-4-one”: Marin, L.; Zhang, Y.; Robeyns, K.; Champagne, B.; Adriaensens, P.; Lutsen, L.; Vanderzande, D.; Bevk, D.; Maes, W. Tetrahedron Lett. 2013, 54, 526–529 (IF2013 2.391) – DOI:10.1016/j.tetlet.2012.11.068
61. “Synthetic protocols towards functionalized selenacalix[3]triazines”: Thomas, J.; Van Rossom, W.; Van Hecke, K.; Van Meervelt, L.; Smet, M.; Dehaen, W.; Maes, W. Synthesis 2013, 45, 734–742 (IF2013 2.443) – DOI:10.1055/s-0032-1318265
60. “Selenacalix[3]triazines: anion versus proton association”: Van Rossom, W.; Thomas, J.; Terentyeva, T. G.; Maes, W.; Dehaen, W. Eur. J. Org. Chem. 2013, 2085–2090 (IF2013 3.154) – DOI:10.1002/ejoc.20139002
59. “Improved thermal stability of bulk heterojunctions based on side-chain functionalized poly(3-alkylthiophene) copolymers and PCBM“: Bertho, S.; Campo, B.; Piersimoni, F.; Spoltore, D.; D’Haen, J.; Lutsen, L.; Maes, W.; Vanderzande, D.; Manca, J. Sol. Energy Mater. Sol. Cells 2013, 110, 69–76 (IF2013 5.030) – DOI:10.1016/j.solmat.2012.12.007
58. “Ester-functionalized poly(3-alkylthiophene) copolymers: synthesis, physicochemical characterization and performance in bulk heterojunction organic solar cells”: Campo, B.; Bevk, D.; Kesters, J.; Gilot, J.; Bolink, H. J.; Zhao, J.; Bolsée, J.-C.; Oosterbaan, W. D.; Bertho, S.; Ruttens, B.; D’Haen, J.; Manca, J.; Lutsen, L.; Van Assche, G.; Maes, W.; Janssen, R. A. J.; Vanderzande, D. Org. Electron. 2013, 14, 523–534 (IF2013 3.676) – DOI:10.1016/j.orgel.2012.11.021
57. “Charge transfer in the weak driving force limit in blends of MDMO-PPV and dithienylthiazolo[5,4-d]thiazoles towards organic photovoltaics with high VOC”: Nevil, N.; Ling, Y.; Van Mierloo, S.; Kesters, J.; Piersimoni, F.; Adriaensens, P.; Lutsen, L.; Vanderzande, D.; Manca, J.; Maes, W.; Van Doorslaer, S.; Goovaerts, E. Phys. Chem. Chem. Phys. 2012, 14, 15774–15784 (IF2012 3.829).
56. “Solvent-dependent deprotonation of meso-pyrimidinylcorroles: absorption and fluorescence studies”: Kruk, M. M.; Ngo, T. H.; Savva, V. A.; Starukhin, A. S.; Dehaen, W.; Maes, W. J. Phys. Chem. A 2012, 116, 10704–10711 (IF2012 2.771).
55. “Unraveling the fluorescence features of individual corrole NH tautomers”: Kruk, M. M.; Ngo, T. H.; Verstappen, P.; Starukhin, A. S.; Hofkens, J.; Dehaen, W.; Maes, W. J. Phys. Chem. A 2012, 116, 10695–10703 (IF2012 2.771).
54. “Corrole NH tautomers: spectral features and individual protonation”: Ivanova, Y. B.; Savva, V. A.; Mamardashvili, N. Zh.; Starukhin, A. S.; Ngo, T. H.; Dehaen, W.; Maes, W.; Kruk, M. M. J. Phys. Chem. A 2012, 116, 10683–10694 (IF2012 2.771).
53. “Homoselenacalix[n]arenes: synthetic exploration and metallosupramolecular chemistry”: Thomas, J.; Dobrzańska, L.; Van Hecke, K.; Sonawane, M. P.; Van Meervelt, L.; Woźniak, K.; Smet, M.; Maes, W.; Dehaen, W. Org. Biomol. Chem. 2012, 10, 6526–6536 (IF2012 3.568).
52. “Corrole-porphyrin conjugates with interchangeable metal centers”: Ngo, T. H.; Nastasi, F.; Puntoriero, F.; Campagna, S.; Dehaen, W.; Maes, W. Eur. J. Org. Chem. 2012, 5605–5617 (IF2012 3.344).
51. “Functionalized dithienylthiazolo[5,4-d]thiazoles for solution-processable organic field-effect transistors”: Van Mierloo, S.; Vasseur, K.; Van den Brande, N.; Boyukbayram, A. E.; Ruttens, B.; Rodriguez, S. D.; Botek, E.; Liégeois, V.; D’Haen, J.; Adriaensens, P. J.; Heremans, P.; Champagne, B.; Van Assche, G.; Lutsen, L.; Vanderzande, D. J.; Maes, W. ChemPlusChem 2012, 77, 923–930 (IF2013 3.242).
50. “TOF-SIMS investigation of degradation pathways occurring in a variety of organic photovoltaic devices – the ISOS-3 inter-laboratory collaboration”: Andreasen, B.; Tanenbaum, D. M.; Hermenau, M.; Voroshazi, E.; Loyd, M. T.; Galagan, Y.; Zimmermann, B.; Kudret, S.; Maes, W.; Lutsen, L.; Vanderzande, D.; Würfel, U.; Andriessen, R.; Rösch, R.; Hoppe, H.; Teran-Escobar, G.; Lira-Cantu, M.; Rivaton, A.; Uzunoğlu, G. Y.; Germack, D.; Hösel, M.; Dam, H. F.; Jørgensen, M.; Gevorgyan, S. A.; Madsen, M. V.; Bundgaard, E.; Krebs, F. C.; Norrman, K. Phys. Chem. Chem. Phys. 2012, 14, 11780–11799 (IF2012 3.829).
49. “On the stability of a variety of organic photovoltaic devices by IPCE and in situ IPCE analyses – the ISOS-3 inter-laboratory collaboration”: Teran-Escobar, G.; Tanenbaum, D. M.; Voroshazi, E.; Hermenau, M.; Norrman, K.; Lloyd, M. T.; Galagan, Y.; Zimmermann, B.; Hösel, M.; Dam, H. F.; Jørgensen, M.; Gevorgyan, S.; Kudret, S.; Maes, W.; Lutsen, L.; Vanderzande, D.; Würfel, U.; Andriessen, R.; Rösch, R.; Hoppe, H.; Rivaton, A.; Uzunoğlu, G. Y.; Germack, D.; Andreasen, B.; Madsen, M. V.; Bundgaard, E.; Krebs, F. C.; Lira-Cantu, M. Phys. Chem. Chem. Phys. 2012, 14, 11824–11845 (IF2012 3.829).
48. “Oligoether-strapped meso-pyrimidinylporphyrins”: Ngo, T. H.; Nitychoruk, K. A.; Lentz, D.; Dehaen, W.; Maes, W. Tetrahedron Lett. 2012, 53, 2406–2409 (IF2012 2.397).
47. “Investigation of the degradation mechanism of a variety of organic photovoltaic devices by combination of imaging techniques – The ISOS-3 inter-laboratory collaboration”: Rösch, R.; Tanenbaum, D. M.; Jørgensen, M.; Seeland, M.; Bärenklau, M.; Hermenau, M.; Voroshazi, E.; Lloyd, M. T.; Galagan, Y.; Zimmermann, B.; Würfel, U.; Hösel, M.; Dam, H. F.; Gevorgyan, S. A.; Kudret, S.; Maes, W.; Lutsen, L.; Vanderzande, D.; Andriessen, R.; Teran-Escobar, G.; Lira-Cantu, M.; Rivaton, A.; Uzunoğlu, G. Y.; Germack, D.; Andreasen, B.; Madsen, M. V.; Norrman, K.; Hoppe, H.; Krebs, F. C. Energy Environ. Sci. 2012, 5, 6521–6540 (IF2012 11.653).
46. “Vibrational States of Zn-meso-Indolo[3,2-b]carbazolyl-Substituted Porphyrins: Fluorescence Line Narrowing Study”: Starukhin, A.; Kruk, M.; Gladkov, L.; Ngo, T. H.; Dehaen, W.; Maes, W.; Scheblykin, I. Vib. Spectrosc. 2012, 61, 199–205 (IF2012 1.747).
45. “Combined experimental-theoretical NMR study on 2,5-bis(5-aryl-3-hexylthiophen-2-yl)thiazolo[5,4-d]thiazole derivatives for printable electronics”: Van Mierloo, S.; Liégeois, V.; Kudrjasova, J.; Botek, E.; Lutsen, L.; Champagne, B.; Vanderzande, D.; Adriaensens, P.; Maes, W. Magn. Reson. Chem. 2012, 50, 379–387 (IF2012 1.528).
44. “(Thio)Ureido anion receptors based on a 1,3-alternate oxacalix[2]arene[2]pyrimidine scaffold”: Van Rossom, W.; Caers, J.; Robeyns, K.; Van Meervelt, L.; Maes, W.; Dehaen, W. J. Org. Chem. 2012, 77, 2791–2797 (IF2012 4.564).
43. “Improved photovoltaic performance of a semi-crystalline narrow bandgap copolymer based on 4H-cyclopenta[2,1-b;3,4-b]dithiophene donor and thiazolo[5,4-d]thiazole acceptor units”: Van Mierloo, S.; Hadipour, A.; Spijkman, M.-J.; Van den Brande, N.; Ruttens, B.; Kesters, J.; D’Haen, J.; Van Assche, G.; de Leeuw, D. M.; Aernouts, T.; Manca, J.; Lutsen, L.; Vanderzande, D.; Maes, W. Chem. Mater. 2012, 24, 587–593 (IF2012 8.238).
42. “Living polymerization via anionic initiation for the synthesis of well-defined PPV materials”: Cosemans, I.; Wouters, J.; Maes, W.; Cleij, T. J.; Lutsen, L.; Junkers, T.; Vanderzande, D. J. M. Macromol. Rapid Commun. 2012, 33, 242–247 (IF2012 4.929).
41. “Selenacalix[3]triazines: synthesis and host-guest chemistry”: Thomas, J.; Van Rossom, W.; Van Hecke, K.; Van Meervelt, L.; Smet, M.; Maes, W.; Dehaen, W. Chem. Commun. 2012, 43–45 (IF2012 6.378).
40. “The ISOS-3 inter-laboratory collaboration focused on the stability of a variety of organic photovoltaic devices”: Tanenbaum, D. M.; Hermenau, M.; Voroshazi, E.; Lloyd, M. T.; Galagan, Y.; Zimmermann, B.; Hösel, M.; Dam, H. F.; Jørgensen, M.; Gevorgyan, S. A.; Kudret, S.; Maes, W.; Lutsen, L.; Vanderzande, D.; Würfel, U.; Andriessen, R.; Rösch, R.; Hoppe, H.; Lira-Cantu, M.; Rivaton, A.; Uzunoğlu, G. Y.; Germack, D.; Andreasen, B.; Madsen, M. V.; Norrman, K.; Krebs, F. C. RSC Adv. 2012, 2, 882–893 (IF2012 2.562).
39. “Thermal Stability of Poly[2-methoxy-5-(2’-phenylethoxy)-1,4-phenylene vinylene] (MPE-PPV):Fullerene Bulk Heterojunction Solar Cells”: Vandenbergh, J.; Conings, B.; Bertho, S.; Kesters, J.; Spoltore, D.; Esiner, S.; Zhao, J.; Van Assche, G.; Wienk, M. M.; Maes, W.; Lutsen, L.; Van Mele, B.; Janssen, R. A. J.; Manca, J.; Vanderzande, D. J. M. Macromolecules 2011, 44, 8470–8478 (IF2011 5.167).
38. “Fingerprints for structural defects in poly(thienylene vinylene) (PTV): A joint theory-experiment NMR study of model molecules”: Diliën, H.; Marin, L.; Botek, E.; Champagne, B.; Lemaur, V.; Beljonne, D.; Lazzaroni, R.; Cleij, T. J.; Maes, W.; Lutsen, L.; Vanderzande, D.; Adriaensens, P. J. J. Phys. Chem. B 2011, 115, 12040–12050 (IF2011 3.696).
37. “Discovery of an anionic polymerization mechanism for high molecular weight PPV derivatives via the sulfinyl precursor route”: Cosemans, I.; Hontis, L.; Van Den Berghe, D.; Palmaerts, A.; Wouters, J.; Cleij, T.; Lutsen, L.; Maes, W.; Junkers, T.; Vanderzande, D. Macromolecules 2011, 44, 7610–7616 (IF2011 5.167).
36. “Determination of the surface acidity of a free-base corrole in a self-assembled monolayer”: Nulens, W.; Grabowska, I.; Ngo, T. H.; Maes, W.; Dehaen, W.; Radecka, H.; Radecki, J. J. Inclusion Phenom. Macrocyclic Chem. 2011, 71, 499–505 (IF2011 1.886).
35. “Homothiacalix[4]arenes: revisiting underexposed macrocycles”: Thomas, J.; Van Hecke, K.; Robeyns, K.; Van Rossom, W.; Sonawane, M. P.; Van Meervelt, L.; Smet, M.; Maes, W.; Dehaen, W. Chem. Eur. J. 2011, 17, 10339–10349 (IF2011 5.925).
34. “Synthesis and characterization of water-soluble poly(p-phenylene vinylene) derivatives via the dithiocarbamate precursor route”: Vandenbergh, J.; Dergent, J.; Conings, B.; Gopala Krishna, T. V. V.; Maes, W.; Cleij, T. J.; Lutsen, L.; Manca, J.; Vanderzande, D. J. M. Eur. Polym. J. 2011, 47, 1827–1835 (IF2011 2.739).
33. “Transformations in the fluorescence line narrowing spectra of porphin upon the formation of diprotonated species”: Starukhin, A. S.; Kruk, M. M.; Gladkova, O. L.; Maes, W. Macroheterocycles 2011, 4, 85–88 (IF2011 0.548).
32. “Influence of macrocycle protonation on the photophysical properties of porphyrins. A review”: Kruk, M. M.; Starukhin, A. S.; Maes, W. Macroheterocycles 2011, 4, 69–79 (IF2011 0.548).
31. “Odd-numbered oxacalix[n]arenes (n = 5, 7): synthesis and solid-state structures”: Van Rossom, W.; Robeyns, K.; Ovaere, M.; Van Meervelt, L.; Dehaen, W.; Maes, W. Org. Lett. 2011, 13, 126–129 (IF2011 5.862).
30. “Luminescence of meso-pyrimidinylcorroles: relationship with substitution pattern and heavy atom effects”: Nastasi, F.; Campagna, S.; Ngo, T. H.; Dehaen, W.; Maes, W.; Kruk, M. Photochem. Photobiol. Sci. 2011, 10, 143–150 (IF2011 2.584).
29. “A three-step synthetic approach to asymmetrically functionalized 4H-cyclopenta[2,1-b:3,4-b’]dithiophenes”: Van Mierloo, S.; Adriaensens, P.; Maes, W.; Lutsen, L.; Cleij, T.; Botek, E.; Champagne, B.; Vanderzande, D. J. Org. Chem. 2010, 75, 7202–7209 (IF2010 4.002).
28. “Synthetic exploration of oxacalix[2]arene[2]quinazolines”: Van Rossom, W.; Kishore, L.; Robeyns, K.; Van Meervelt, L.; Dehaen, W.; Maes, W. Eur. J. Org. Chem. 2010, 4122–4129 (IF2010 3.206).
27. “Synthetic, structural and photophysical exploration of meso-pyrimidinyl-substituted AB2-corroles”: Ngo, T. H.; Puntoriero, F.; Nastasi, F.; Robeyns, K.; Van Meervelt, L.; Campagna, S.; Dehaen, W.; Maes, W. Chem. Eur. J. 2010, 16, 5691–5705 (IF2010 5.476).
26. “meso-Indolo[3,2-b]carbazolyl-substituted porphyrinoids: synthesis, characterization and effect of the number of indolocarbazole moieties on the photophysical properties”: Maes, W.; Ngo, T. H.; Rong, G.; Starukhin, A. S.; Kruk, M. M.; Dehaen, W. Eur. J. Org. Chem. 2010, 2576–2586 (IF2010 3.206).
25. “An oxacalix[2]arene[2]pyrimidine-bis(Zn-porphyrin) tweezer as a selective receptor towards fullerene C70”: Van Rossom, W.; Kundrát, O.; Ngo, T. H.; Lhoták, P.; Dehaen, W.; Maes, W. Tetrahedron Lett. 2010, 51, 2423–2426 (IF2010 2.618).
24. “meso-Pyrimidinyl-substituted A2B- and A3-corroles”: Ngo, T. H.; Nastasi, F.; Puntoriero, F.; Campagna, S.; Dehaen, W.; Maes, W. J. Org. Chem. 2010, 75, 2127–2130 (IF2010 4.002).
23. “Determination of interaction strength between corrole and phenol derivatives in aqueous media using atomic force microscopy”: Szymanska, I.; Dolusic, E.; Dehaen, W.; Maes, W.; Ito, T.; Radecka, H. Supramol. Chem. 2009, 21, 555–563 (IF2009 1.885).
22. “Expeditive syntheses of functionalized pentahelicenes and NC-AFM on Ag (001)”: Goretta, S.; Tasciotti, C.; Raimundo, J.-M.; Mathieu, S.; Smet, M.; Maes, W.; Chabre, Y. M.; Dehaen, W.; Giasson, R.; Henry, C.; Barth, C.; Gingras, M. Org. Lett. 2009, 11, 3846–3849 (IF2009 5.420).
21. “Synthetic aspects of porphyrin dendrimers”: Maes, W.; Dehaen, W. Eur. J. Org. Chem. 2009, 4719–4752 (IF2009 3.096).
20. “Homoselenacalix[n]arenes”: Thomas, J.; Maes, W.; Robeyns, K.; Ovaere, M.; Van Meervelt, L.; Smet, M.; Dehaen, W. Org. Lett. 2009, 11, 3040–3043 (IF2009 5.420).
19. “Efficient fragment coupling approaches toward large oxacalix[n]arenes (n = 6, 8)”: Van Rossom, W.; Ovaere, M.; Van Meervelt, L.; Dehaen, W.; Maes, W. Org. Lett. 2009, 11, 1681–1684 (IF2009 5.420).
18. “Reductive demetallation of Cu-corroles - A new protective strategy towards functional free-base corroles”: Ngo, T. H.; Van Rossom, W.; Dehaen, W.; Maes, W. Org. Biomol. Chem. 2009, 7, 439–443 (IF2009 3.762).
17. “Oxacalix[n](het)arenes”: Maes, W.; Dehaen, W. Chem. Soc. Rev. 2008, 37, 2393–2402 (IF2008 17.419).
16. “PVC supported liquid membrane and carbon paste potentiometric sensors incorporating a Mn(III)-porphyrin for direct determination of undissociated paracetamol”: Saraswathyamma, B.; Pajak, M.; Radecki, J.; Maes, W.; Dehaen, W.; Kumar, K. G.; Radecka, H. Electroanalysis 2008, 20, 2009–2015 (IF2008 2.901).
15. “Macrostructures derived from dichloropyrimidines”: Maes, W.; Dehaen, W. Pol. J. Chem. 2008, 82, 1145–1160 (IF2008 0.518).
14. “Efficient post-macrocyclization functionalizations of oxacalix[2]arene[2]pyrimidines“: Van Rossom, W.; Maes, W.; Kishore, L.; Ovaere, M.; Van Meervelt, L.; Dehaen, W. Org. Lett. 2008, 10, 585–588 (IF2008 5.128).
13. “meso-Pyrimidinyl-substituted A2B-corroles“: Maes, W.; Ngo, T. H.; Vanderhaeghen, J.; Dehaen, W. Org. Lett. 2007, 9, 3165–3168 (IF2007 4.802).
12. “Efficient synthesis of aryldipyrromethanes in water and their application in the synthesis of corroles and dipyrromethenes”: Rohand, T.; Dolusic, E.; Ngo, T. H.; Maes, W.; Dehaen, W. ARKIVOC 2007, (x), 307–324 (IF2007 1.253).
11. “Salicylate determination in human plasma by ISEs incorporating Mn(III)-porphyrin and Zn(II)-dipyrromethene”: Radecka, H.; Grzybowska, I.; Radecki, J.; Jakubowski, P.; Loteran, S.; Orlewska, C.; Maes, W.; Dehaen, W. Anal. Lett. 2007, 40, 387–401 (IF2007 1.362).
10. “Selective synthesis of functional oxa- and thiacalix[2]arene[2]pyrimidines“: Maes, W.; Van Rossom, W.; Van Hecke, K.; Van Meervelt, L.; Dehaen, W. Org. Lett. 2006, 8, 4161–4164 (IF2006 4.659).
9. “Synthesis of multi(metallo)porphyrin dendrimers through nucleophilic aromatic substitution on meso-pyrimidinyl substituted porphyrins“: Maes, W.; Vanderhaeghen, J.; Smeets, S.; Asokan, C. V.; Van Renterghem, L. M.; Du Prez, F. E.; Smet, M.; Dehaen, W. J. Org. Chem. 2006, 71, 2987–2994 (IF2006 3.790).
8. “Synthesis of 1,2,4-triazole dendrimers“: Maes, W.; Verstappen, B.; Dehaen, W. Tetrahedron 2006, 62, 2677–2683 (IF2006 2.817).
7. “Porphyrin-functionalized dendrimers: synthesis and application as recyclable photocatalysts in a nanofiltration membrane reactor“: Chavan, S. A.; Maes, W.; Gevers, L. E. M.; Wahlen, J.; Vankelecom, I. F. J.; Jacobs, P. A.; Dehaen, W.; De Vos, D. E. Chem. Eur. J. 2005, 11, 6754–6762 (IF2005 4.907).
6. “5,5-Dialkyldipyrromethane as a precursor for the synthesis of calix[4]phyrins and pseudocorroles using MacDonald [2+2] condensations“: Orlewska, C.; Maes, W.; Toppet, S.; Dehaen, W. Tetrahedron Lett. 2005, 46, 6067–6070 (IF2005 2.477).
5. “meso-Dichloropyrimidinyl substituted expanded porphyrins“: Maes, W.; Vanderhaeghen, J.; Dehaen, W. Chem. Commun. 2005, 2612–2614 (IF2005 4.426).
4. “Benzimidazole-functionalized dendrons as molybdenum supports for selective epoxidation catalysis“: Chavan, S.; Maes, W.; Wahlen, J.; Jacobs, P.; De Vos, D.; Dehaen, W. Catal. Commun. 2005, 6, 241–246 (IF2005 2.098).
3. “Synthesis of novel dendrimers containing pyrimidine units“: Maes, W.; Amabilino, D. B.; Dehaen, W. Tetrahedron 2003, 59, 3937–3943 (IF2003 2.641).
2. “Sterically encumbered porphyrins by Suzuki reactions of a 5,15-bis(4,6-dichloropyrimidin-5-yl) derivative“: Maes, W.; Dehaen, W. Synlett 2003, 79–82 (IF2003 2.741).
1. “The use of 1,3,5-triazines in dendrimer synthesis“: Verheyde, B.; Maes, W.; Dehaen, W. Mater. Sci. Eng.,C 2001, 18, 243–245 (IF2001 0.905).

Since December 2020, the DSOS group has moved into the brand new Science Tower at UHasselt, together with all other MATCHEM colleagues! Take a look at the imo-imomec website to know more about our infrastructure and equipment.

